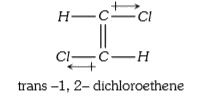
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Zero dipole moment is present in
Text Solution
|
- The dipole moment is zero for the compound
Text Solution
|
- Dipole moment will be zero in the complexes
Text Solution
|
- जल का द्विध्रुव आघूर्ण शून्य होता है।
Text Solution
|
- Define the dipole moment. Why the BF3 molecule dipole moment is zero?
Text Solution
|
- Zero dipole moment is present in
Text Solution
|
- Whose dipole moment is zero-
Text Solution
|
- शून्य द्विध्रुव आघूर्ण वाला यौगिक है
Text Solution
|
- whose dipole moment is not zero
Text Solution
|