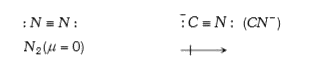A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- N(2) is less reactive than CN^(-) due to
Text Solution
|
- The species CO, CN^(-) ,and NO^(+) which are isoelectronic with N(2) ,...
Text Solution
|
- Why is N(2) less reactive at room temperature ?
Text Solution
|
- Assertion : Nitrogen is less reactive than molecular oxygen. Reason: B...
Text Solution
|
- Why is N(2) less reactive at room temperature ?
Text Solution
|
- Why is N(2) less reactive at room temperature ?
Text Solution
|
- Assertion. Nitrogen is less reactive than molecular oxygen. Reason. ...
Text Solution
|
- N(2) कमरे के ताप पर कम क्रियशील क्यों है ?
Text Solution
|
- Why is N(2) less reactive at room temperature ?
Text Solution
|