A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Dipole moment of is 1.5D. The dipole moment of
Text Solution
|
- Dipole moment of is 1.5D. The dipole moment of
Text Solution
|
- The dipole moment of is 1.5D what will be the dipole moment of
Text Solution
|
- If the dipole moment of the following molecule is 1.5D Then the dip...
Text Solution
|
- The dipole moment of is 1.5 D. The dipole moment of is
Text Solution
|
- Dipole Moment,determination of shape based on dipole oment,magnitude o...
Text Solution
|
- The dipole moment of is 1.5 D. The dipole Moment of in Debye
Text Solution
|
- The dipole moment of is 1.5 D. The dipole moment of is:
Text Solution
|
- The dipole moment of is 1.5D. The dipole moment of is :
Text Solution
|
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of 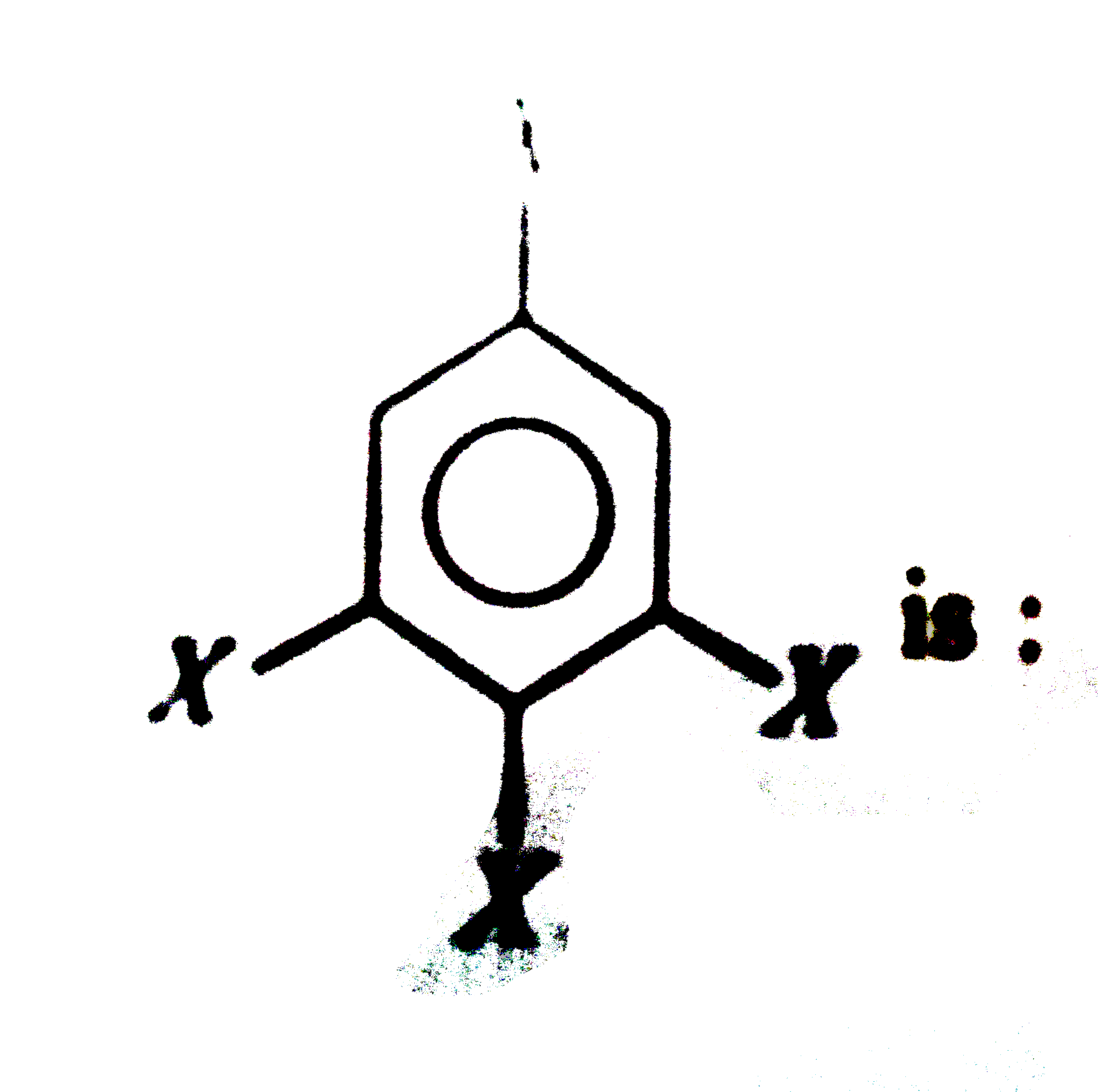
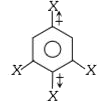 , The bond moments at para positions cancel due to opposite direction. The other two groups are meta to each other, thus angle between the two bond moments is 120° and hence,`mu = 1.5D.`
, The bond moments at para positions cancel due to opposite direction. The other two groups are meta to each other, thus angle between the two bond moments is 120° and hence,`mu = 1.5D.`