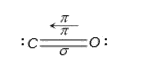A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CO is practically non-polar since
Text Solution
|
- CO is practically non-polar since
Text Solution
|
- CO is practically non-polar since-
Text Solution
|
- CO(2) is non-polar but COS is polar, even though both of them are line...
Text Solution
|
- Non-polar compounds are soluble in non -polar solvents
Text Solution
|
- Assertion (A): H(2), Li(2), C(2), N(2) are diamagnetic. Reason (R): ...
Text Solution
|
- What are polar and non-polar molecules?
Text Solution
|
- Classify the following molecules into Polar and non-polar. (H2O, H2, N...
Text Solution
|
- CO व्यवहारिक रूप से अध्रुवीय होता है जब
Text Solution
|