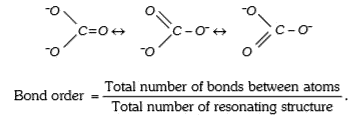
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The bond order in CO3^(2-) ion between C - O is
Text Solution
|
- The correct roder o fincreasing C-O bond lengths in CO, CO3^(2-) and ...
Text Solution
|
- The bond order in CO(2)^(2-) ion between C - O is
Text Solution
|
- Bond order of N-O bonds in nitrate ion is
Text Solution
|
- Cl-O bond order in perchlorate ion is
Text Solution
|
- What is the bond order between C and O in CO ?
Text Solution
|
- The bond order of the N-O bonds in NO3^- ion is
Text Solution
|
- CO3^(2-) आयन में C-O के बीच बन्ध क्रम है।
Text Solution
|
- The Cl-O bond order in perchlorate ion
Text Solution
|