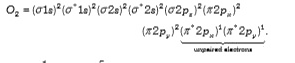A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- According to molecular orbital theory, the paramagnetism of O(2) molec...
Text Solution
|
- According to molecular orbital theory, which of the following will not...
Text Solution
|
- According to molecular orbital theory, the paramagnetism of O(2) molec...
Text Solution
|
- According to molecular orbital theory
Text Solution
|
- आणविक ऑर्बिटलों सिद्धान्त के अनुसार O(2) अणु में कितने अयुग्मित इले...
Text Solution
|
- आणविक ऑर्बिटलों आरेख के आधार पर स्पष्ट कीजिए कि O(2) अणु की प्रकृति ...
Text Solution
|
- According to the molecular orbitals theory , O(2)^(+) possesses
Text Solution
|
- According to Molecular Orbital Theory,
Text Solution
|
- Using Molecular Orbital Theory explain why the B(2) molecule is parama...
Text Solution
|