A
B
C
D
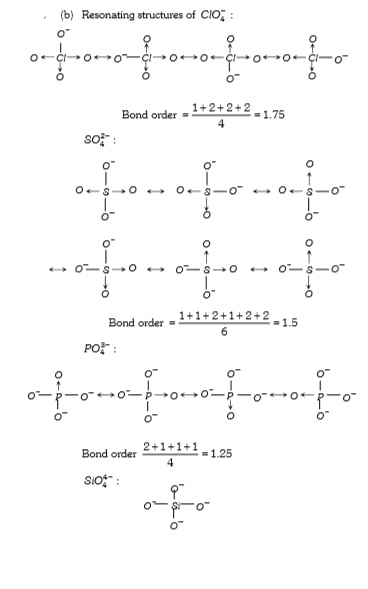
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following ions in the order of decreasing X-O bond length ...
Text Solution
|
- Arrange the following in order of decreasing N - O bond length NO(2)^(...
Text Solution
|
- Arrange the following ions in the order of decreasing X-O bond length ...
Text Solution
|
- Arrange the following in decreasing order of their C=C bond length
Text Solution
|
- Arrange the following molecules in the correct order of decreasing C-C...
Text Solution
|
- Arrange the following ions in the order of decreasing X -O bond length...
Text Solution
|
- Which one of the following molecule will have all equal X-F bonds leng...
Text Solution
|
- Arrange O (2) H(2)O(2) and O (3) in the correct order of decreasing O-...
Text Solution
|
- Which one of the following molecule will have all equal X-F bonds leng...
Text Solution
|