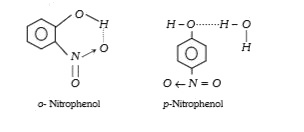
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- o-nitrophenol is more volatile than p-nitrophenol it is due to
Text Solution
|
- Out of o-nitrophenol and p-nitrophenol, which is more volatile ? Expla...
Text Solution
|
- Which of the following is more volatile : o-nitrophenol or p-nitrophen...
Text Solution
|
- Out of o-nitrophenol annd p-nitrophenol, which is more volatile? Expla...
Text Solution
|
- Account for the following: o-nitrophenol is more steam volatile than p...
Text Solution
|
- O-नाइट्रोफीनॉल तथा p-नाइट्रोफीनॉल में से कौन-सा अधिक वाष्पशील है?
Text Solution
|
- O-Nitrophenol is more volatile than p-Nitrophenot (Or) boling point of...
Text Solution
|
- Which of the following isomers is more volatile: o-nitrophenol or p-ni...
Text Solution
|
- Why o - Nitrophenol is more volatile than p - Nitrophenol.
Text Solution
|