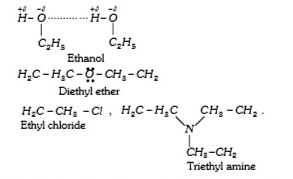
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydrogen bonding is maximum in
Text Solution
|
- Hydrogen bonding is maximum in:
Text Solution
|
- Hydrogen bonding is maximum in
Text Solution
|
- Hydrogen bonding is maximum in
Text Solution
|
- अधिकतम हाइड्रोजन बन्धता है :
Text Solution
|
- Hydrogen bonding is maximum in
Text Solution
|
- Hydrogen bonding is maximum in
Text Solution
|
- Hydrogen bonding is maximum in
Text Solution
|
- Hydrogen bonding is maximum in:
Text Solution
|