A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
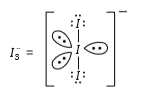
- Total number of lone pair of electrons in 3 I(3)^(-) ion is
Text Solution
|
- The hybridisation number of lone pair of electron and shape of I(3)^(Θ...
Text Solution
|
- The total number of lone pair of electrons in N(2)O(3) is
Text Solution
|
- Total number of lone pair of electrons in 3 I(3)^(-) ion is
Text Solution
|
- Total number of lone pairs of electrons in I(3)^(-) ion is
Text Solution
|
- The total number of lone pairs of electrons in N(2)O(3) is
Text Solution
|
- The total number of lone pairs of electrons in N(2)O(3) is
Text Solution
|
- Total number of lone pair of electrons I(3)^(-) ion is-
Text Solution
|
- I(3)^(-)आयन में इलेक्ट्रॉनो के एकाकी युग्म की कुल संख्या होगी।
Text Solution
|