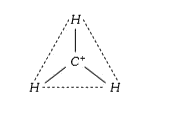A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The species in which the central atom uses sp^2 hybrid orbitals in its...
Text Solution
|
- The species in which the cantral atom uses sp^(2) hybrid orbital in it...
Text Solution
|
- In which of the following the central atom does not use sp^(2) hybrid ...
Text Solution
|
- In which of the following central atom makes use of sp^(3) hybrid orbi...
Text Solution
|
- A molecule in which sp^2 hybrid orbitals are used by the central atom ...
Text Solution
|
- In which of the following molecules the central atom does not use sp^3...
Text Solution
|
- In which of the following the central atom does not use sp^3 hybrid or...
Text Solution
|
- In which of the following the central atoms does not use sp^(3) hybrid...
Text Solution
|
- The species in which the central atom uses sp^(2) hybrid orbitals in i...
Text Solution
|