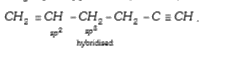A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the organic compound CH2 = CH- CH2 - CH2 - C -= CH, the pair of hyb...
Text Solution
|
- In the organic compound CH3==CH-CH2-CH2--=CH , the parh of hydridised ...
Text Solution
|
- The hybridisation of carbons involved in C-C single bond in CH-=C-CH =...
Text Solution
|
- In the compound CH2=CH-CH2-CH2-C-=CH , the C2-C3 bond is of the type
Text Solution
|
- यौगिक CH2=CH-CH2-CH2-C-=CH में C2-C3 बन्ध का प्रकार है :
Text Solution
|
- Write the IUPAC names of the following compounds CH3 -CH =CH- CH2 -CH=...
Text Solution
|
- CH2 = CH-CH2-CH2-C The hybrid cells that form the bonding -2 -c -3 in ...
Text Solution
|
- In the organic compound CH2 = CH- CH2 - CH2 - C -= CH, the pair of hyb...
Text Solution
|
- यौगिक CH2=CH - CH2 - CH2 -C -=CH का IUPAC नाम है
Text Solution
|