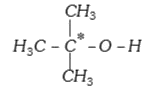
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compound in which carbon uses sp^(3) hybridization for bond format...
Text Solution
|
- The compound with C uses in the sp^(3) hybrid orbitals for bond format...
Text Solution
|
- The compound in which underlined carbon uses only its sp^(3)-hybrid or...
Text Solution
|
- The compound in which all carbon atoms use only sp^(3)-hybrid orbitals...
Text Solution
|
- The compound in which all carbon atoms use only sp^(3)-hybrid orbitals...
Text Solution
|
- The compound in which carbon uses sp^(3) hydrid orbitals for bond form...
Text Solution
|
- The compound in which carbon uses sp^(3) hydrid orbitals for bond form...
Text Solution
|
- The compound in which C* uses sp^(3) hybrid orbitals for bond formatio...
Text Solution
|
- In which of the following, carbon uses sp^3 hybrid orbitals only for b...
Text Solution
|