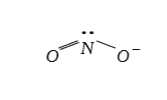
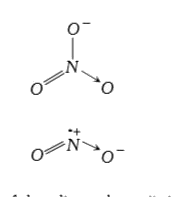
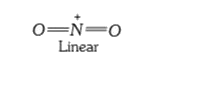A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- Which are the species in which central atom undergoes sp^(3) hybridisa...
Text Solution
|
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- The species in which the N atom is in a state of sp hybridisation is
Text Solution
|
- In which of the following molecule or ion the hybridisation state ofo ...
Text Solution
|
- In which of the following N atom is not sp^(2) hybridised?
Text Solution
|
- The species in which the N atom is in a state of sp hybridization is -
Text Solution
|
- Which of the following species contains 5 H atoms , sp ^2 hybridised N...
Text Solution
|
- वह स्पीशीज जिसमे N-परमाणु sp-संकरण की अवस्था में है, होगी
Text Solution
|