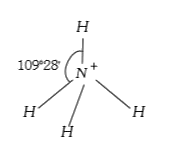
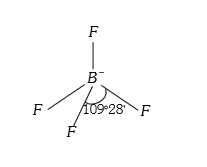
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following species the interatomic bond angle is 109^(@...
Text Solution
|
- In which of the following pairs , bond angle is 109^@28' ?
Text Solution
|
- What of the following triatiomic polar species have bond angle greater...
Text Solution
|
- The bond angle in ammonia is 109^(@)28.
Text Solution
|
- The species having bond angles of 109^(@)28 is .
Text Solution
|
- In which of the following species the interatomic bond angle is 109^(@...
Text Solution
|
- निम्न में से किसमे सभी बंध कोण 109^(@)28' के बराबर हैं ?
Text Solution
|
- All bond angles are exactly equal to 109^(@) 28' in:
Text Solution
|
- निम्न में से किस प्रजाति में अन्तरआण्विक बंधकोण 109^@28' का है
Text Solution
|