A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
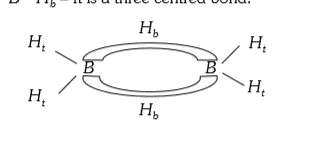
- The two types of bonds present in B(2)H(6) are covalent and .
Text Solution
|
- The two types of bonds present in B(2)H(6) are covalent and .
Text Solution
|
- The bond present in borazole(B(3)N(3)H(6)) are
Text Solution
|
- Two types of carbon- carbon covalent bond lengths are present in
Text Solution
|
- (a) What are covalent bonds? Write the type of bonds present in N(2) a...
Text Solution
|
- What is a covalent bond ? How many covalent bonds are present in the f...
Text Solution
|
- B(2)H(6), में दो प्रकार के बंधनों में एक सहसंयोजन बंधन हो जाता है तथा ...
Text Solution
|
- B(2)H(6) में सहसंयोजक तथा ....... प्रकार के बन्ध उपस्थित होते हैं ।
Text Solution
|
- B(2)H(6) में सहसंयोजक तथा दो प्रकार के बन्ध पाये जाते है |
Text Solution
|