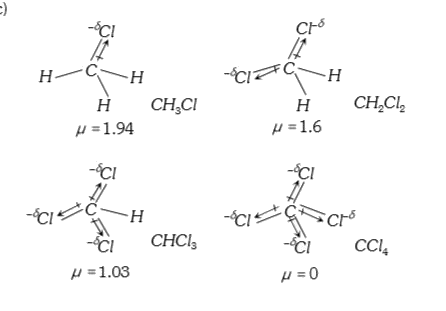A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The order of dipole moment of the following molecules is
Text Solution
|
- The order of dipole moment of the following molecules is
Text Solution
|
- The correct order of dipole moment for the following molecules is
Text Solution
|
- Which of the following is correct order of order of dipole moment :-
Text Solution
|
- The correct order of dipole moment for the following molecules is
Text Solution
|
- The correct order of dipole moment for the following molecules is
Text Solution
|
- Dipole moment order of which of the following pairs of molecules is no...
Text Solution
|
- The molecules that will have dipole moment are
Text Solution
|
- निम्न अणुओं के द्विध्रुव आघूर्ण का क्रम है
Text Solution
|