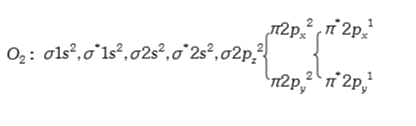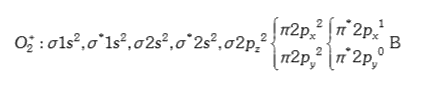A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- According to molecular orbital theory, which of the following statemen...
Text Solution
|
- According to MOT whch of the following statement about magnetic charac...
Text Solution
|
- According to molecular orbital theory which of the following is correc...
Text Solution
|
- According to molecular orbital theory, which of the following statemen...
Text Solution
|
- Using molecular orbital theory, compare the bond energy and magnetic c...
Text Solution
|
- Bond order is a concept in the molecular orbital theory. It depends on...
Text Solution
|
- The bond order depends on the number of electrons in the bonding and a...
Text Solution
|
- According to molecular orbital theory, which of the following statemen...
Text Solution
|
- According to MO theory, which of the following statements about the ma...
Text Solution
|