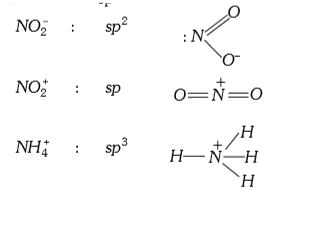
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The hybridisations of atomic orbitals of nitrogen in NO2, NO3^(-) and ...
Text Solution
|
- The hybridisation of atomic orbitals of nitrogen in NO2^+ , NO3^- and...
Text Solution
|
- The nature of N-atomic hybridization in NO3 ^, NO2 + and NH4 + is-
Text Solution
|
- NO2 +, NO3 ^ -, NH4 + The hybridization of N-atoms in ions is, respect...
Text Solution
|
- NO2^(+),NO3^(-)" व "NH4^(+) Have hybridized orbitals, respectively.
Text Solution
|
- The types of hybrid orbitals of nitrogen in NO2^+ NO3^- and NH4^+ resp...
Text Solution
|
- The hybridisation of orbitals of N atom in NO3^(-), NO2^(+) and NH4^(+...
Text Solution
|
- The hybridisation of orbitals of N atom in NO3^(-), NO2^(+) and NH4^(+...
Text Solution
|
- The hybridisation of atomic orbitals of nitrogen in NO2^+ , NO3^- and ...
Text Solution
|