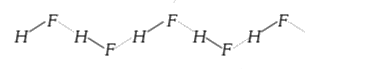A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Given below are two statements : one is labelled as Assertion A and th...
Text Solution
|
- Statement-1 : Dipole-dipole interaction is only non covalent force of ...
Text Solution
|
- Given below are two statements : one is labelled as Assertion A and th...
Text Solution
|
- Given below are two statements : one is labelled as Assertion A and th...
Text Solution
|
- STATEMENT - 1 : When a rod lying freely is heated, no thermal stress i...
Text Solution
|
- Given below are two statement : one is labelled as Assertion A and the...
Text Solution
|
- Given below are two statements, one is labelled as Assertion (A) and o...
Text Solution
|
- Given below are two statements : One is labelled as Assertion A and th...
Text Solution
|
- Given below are two statements labelled as Assertion (A) and Reason (R...
Text Solution
|