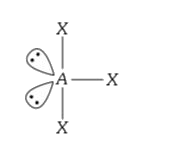
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A central atom in a molecule has two lone pairs of electrons and forms...
Text Solution
|
- Shape Of Molecules In Which Central Atom Has No Lone Pair
Text Solution
|
- Hybridisation And Shapes Of Molecules Containing Bond Pairs And Lone P...
Text Solution
|
- In which of the following molecules, the central atom has one lone pai...
Text Solution
|
- If the central atom in certain molecule has two lone pairs and three b...
Text Solution
|
- A molecule has 3 bonded pairs and 2 lone pairs on the central atom. Ex...
Text Solution
|
- Which of the following molecules has one lone pair of electrons on the...
Text Solution
|
- The shape of the molecule containing 3 bond pairs and one lone pair ar...
Text Solution
|
- The shape of the molecule containing 3 bond pairs and one lone pairs a...
Text Solution
|