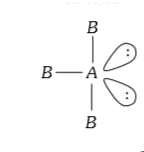
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An inter halogen compound AB3 has T shaped structure, how many lone pa...
Text Solution
|
- How many lone pairs are present in nitrogen molecule ?
Text Solution
|
- How many lone pairs are present in white phosphorous ?
Text Solution
|
- How many lone pairs are present in OF2 molecule ?
Text Solution
|
- In the inter halogen compounds of AB3//AB5 form which is correct:
Text Solution
|
- Assertion : ClF(3) has T-shape structure. Reason : It has two lone p...
Text Solution
|
- AX(5) अन्तर हैलोजन यौगिक की आकृति ……….. होती है|
Text Solution
|
- AX(3) प्रकार के अन्तर हैलोजन यौगिक की आकृति क्या होती है?
Text Solution
|
- Explain the structure of inter halogen compounds.
Text Solution
|