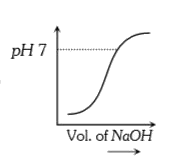
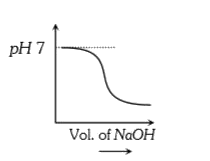
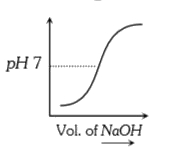
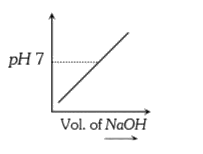A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 100 mL of 0.1 HCl is taken in a beaker and to it 100 mL of 0.1 M NaOH...
Text Solution
|
- 100 ml of 0.1 M CH(3)COOH are mixed with 100ml of 0.1 M NaOH , the pH ...
Text Solution
|
- What is the pH of solution in which 25.0 mL of 0.1 M NaOH is added to ...
Text Solution
|
- When 100 mL of 0.1 M NaCN solution is titrated with 0.1 M HCl solution...
Text Solution
|
- On adding which of the following the pH of 20 ml of 0.1 NaOH will not ...
Text Solution
|
- Calculate the pH of a solution which contains 100 ml of 0.1 H HCl and ...
Text Solution
|
- Calculate the pH of a solution which contains 9.9 mL of 1 M HCl and 10...
Text Solution
|
- To a 50 ml of 0.1 M HCl solution , 10 ml of 0.1 M NaOH is added and t...
Text Solution
|
- 100 mL of 0.1 HCl is taken in a beaker and to it 100 mL of 0.1 M NaOH...
Text Solution
|