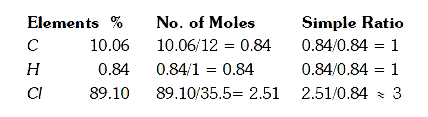A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Percentage composition of an organic compounds is as follows: C = 10.0...
Text Solution
|
- An organic compound on analysis gave the following percentage composit...
Text Solution
|
- An organic compound contains 49.3 % carbon,6.84 % hydrogen and its vap...
Text Solution
|
- An organic compound on analysis was found to contain 10.06 % Carbon,0....
Text Solution
|
- Precentage composition of an organic compounds is as follows, C = 10.0...
Text Solution
|
- The molecular mass of an organic compound is 78 and its percentage com...
Text Solution
|
- An organic compound on analysis gave the following percentage composit...
Text Solution
|
- An organic compound containing carbon, hydrogen and oxygen gave the fo...
Text Solution
|
- एक कार्बनिक यौगिक मे अवयवों के प्रतिशत निम्न है C=10.06, H=0.84 , Cl=8...
Text Solution
|