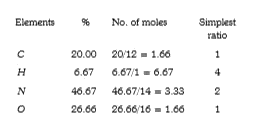A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound having molecular mass 60 is found to contain C = 2...
Text Solution
|
- An organic compound having molecular mass 60 is found to contain C = 2...
Text Solution
|
- An organic compound having molecular mass 60 is found to be contain C=...
Text Solution
|
- An organic compound x having molecular mass 60 is found to contain C =...
Text Solution
|
- An organic compound containing oxygen, carbon, Hydrogen and nitroge...
Text Solution
|
- An organic compound having molecular mass 60 is found to contain C = 2...
Text Solution
|
- एक यौगिक A ग़र्म करने पर रंगहीन गैस तथा एक अपद्रव्य भी देता हैं जिसे पा...
Text Solution
|
- एक कार्बनिक यौगिक जिसका अणुभार 60 है इसमें C=20%, H= 6.67% तथा N= 46.6...
Text Solution
|
- An organic compound having molecular mass 60 is fond to contain C=20%,...
Text Solution
|