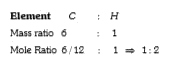A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ration of mass per cent of C and H of an organic compound (C(x)H(y...
Text Solution
|
- A compound (X) with the molecular formula C(3)H(8)O can be oxidized to...
Text Solution
|
- The ratio of mass percent of C and H of an organic compound (C(X)H(Y)O...
Text Solution
|
- The ration of mass per cent of C and H of an organic compound (C(x)H(y...
Text Solution
|
- C(3)H(8)O अणुसूत्र वाले यौगिक X को C(3)H(6)O(2) अणुसूत्र वाले यौगिक Y ...
Text Solution
|
- The ration of mass per cent of C and H of an organic compound (C(x)H(y...
Text Solution
|
- The ratio of mass percent of C and H of an organic compound (C(X) H(Y)...
Text Solution
|
- In a compound C(x)H(y)O(z) , the mass % of C and H is 6:1 and the amou...
Text Solution
|
- किसी कार्बनिक यौगिक (C(x)H(y)O(z)) में C तथा H का द्रव्यमान प्रतिशत 6:...
Text Solution
|