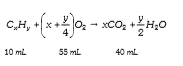A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- At 300 K and 1 atmospheric pressure, 10 mL of a hydrocarbon required 5...
Text Solution
|
- 10 ml of hydrocarbon requries 55 ml of oxygen for complete combustion ...
Text Solution
|
- At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air...
Text Solution
|
- At 300 K and 1 atm, 15 mL of gaseous hydrocarbon requires 375 mL air c...
Text Solution
|
- At 300 K and 1 atmospheric pressure, 10 mL of a hydrocarbon required 5...
Text Solution
|
- 10 mL of a certain hydrocarbon require 25 mL of oxygen for complete co...
Text Solution
|
- 300 K तथा 1 वायुमण्डलीय दाब पर , हाइड्रोकार्बन के 10 mL के पूर्ण दहन...
Text Solution
|
- 10 ml of hydrocarbon requries 55 ml of oxygen for complete combustion ...
Text Solution
|
- At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air...
Text Solution
|