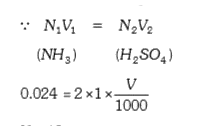Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 0.8 g of an organic compound was analysed by Kjeldahl's method for the...
Text Solution
|
- In Kjeldahl's method used for estimation of nitrogen, ammonia evolved ...
Text Solution
|
- During estimation of nitrogen present in an organic compound by Kjelda...
Text Solution
|
- During estimation of nitrogen present in an organic compound by Kjelda...
Text Solution
|
- During nitrogen estimation present in an organic compound by Kjeldahl'...
Text Solution
|
- During nitrogen estimation present in an organic compound by Kjeldahl'...
Text Solution
|
- During estimation of nitrogen present in an organic compound by Kjelda...
Text Solution
|
- During estimation of nitrogen present in an organic compound by Kjelda...
Text Solution
|
- During estimation of nitrogen present in an organic compound by Kjelda...
Text Solution
|