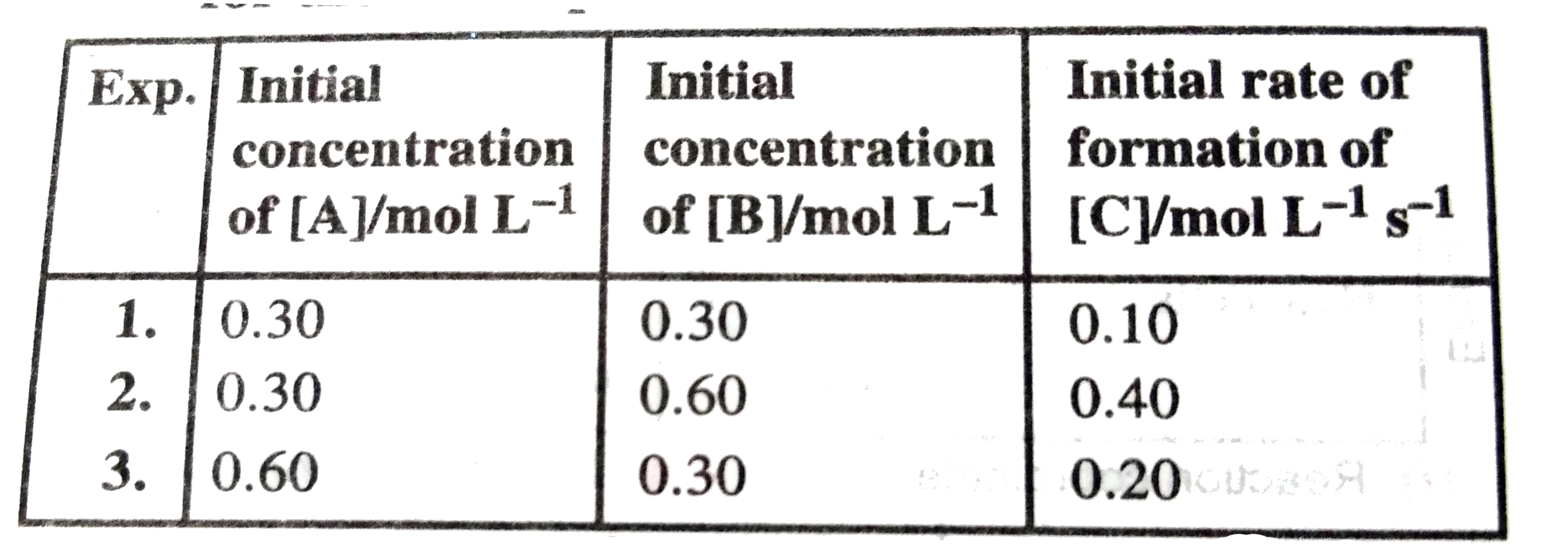
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compounds 'A' and 'B' react according to the following chemical equati...
Text Solution
|
- Compounds A and B react according to the following chemical equation :...
Text Solution
|
- Compounds A and B react according to the following chemical equation :...
Text Solution
|
- Compounds 'A' and 'B' react according to the following chemical equati...
Text Solution
|
- Compounds 'A' and 'B' react according to the following chemical eq...
Text Solution
|
- Compound 'A' and B react according to the following chemical equation....
Text Solution
|
- Rate law for the reaction, A + 2B to C is found to be Rate = k [A] [...
Text Solution
|
- Rate law for the reaction A+2BrarrC , is found to be Rate =k [A][B]...
Text Solution
|
- Compounds 'A' and 'B' react according to the following chemical eq...
Text Solution
|