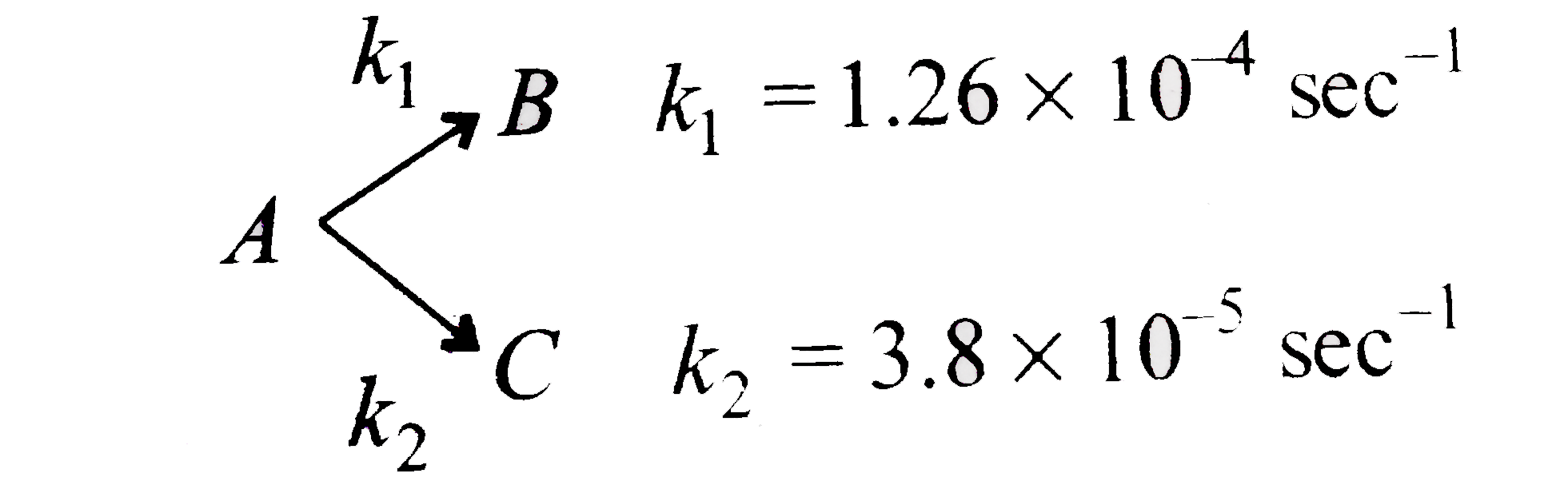
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- A subtance undergoes first order decomposition involving two parallel ...
Text Solution
|
- Consider the following first order decomposition and the accompanying ...
Text Solution
|
- A substance undergoes first order decomposition. It follows two first ...
Text Solution
|
- A substance '' was found to undergo two parallel first order reaction ...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- Psedudo first order reation| parallel elementary reaction| decompositi...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|