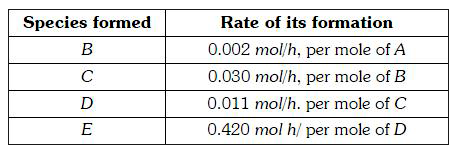
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Given the hypothetical reaction mechanism Aoverset(I)(to)B overset(I...
Text Solution
|
- In honeybees, females are overset("(i)")"" having overset("(ii)")"" ch...
Text Solution
|
- Rate of the given reaction, (1) A + B overset(r(1)=0.05) to X (2) ...
Text Solution
|
- Identify unknown compounds A to E in the following senes of chemical r...
Text Solution
|
- The mechanism of the reaction A+2B rarr C+D is: Step I: A+B underset(k...
Text Solution
|
- Statement -1: In A overset(k(1))to B overset(k(2))to C If half life of...
Text Solution
|
- The stability of carbanions in the following (i) RC-=overset(-"*")C (i...
Text Solution
|
- In the sequence of reaction A overset(K(1)) to B overset(K(2))to C o...
Text Solution
|
- अभिक्रिया की क्रियाविधि A overset(r ) to B overset(II) to C overset...
Text Solution
|