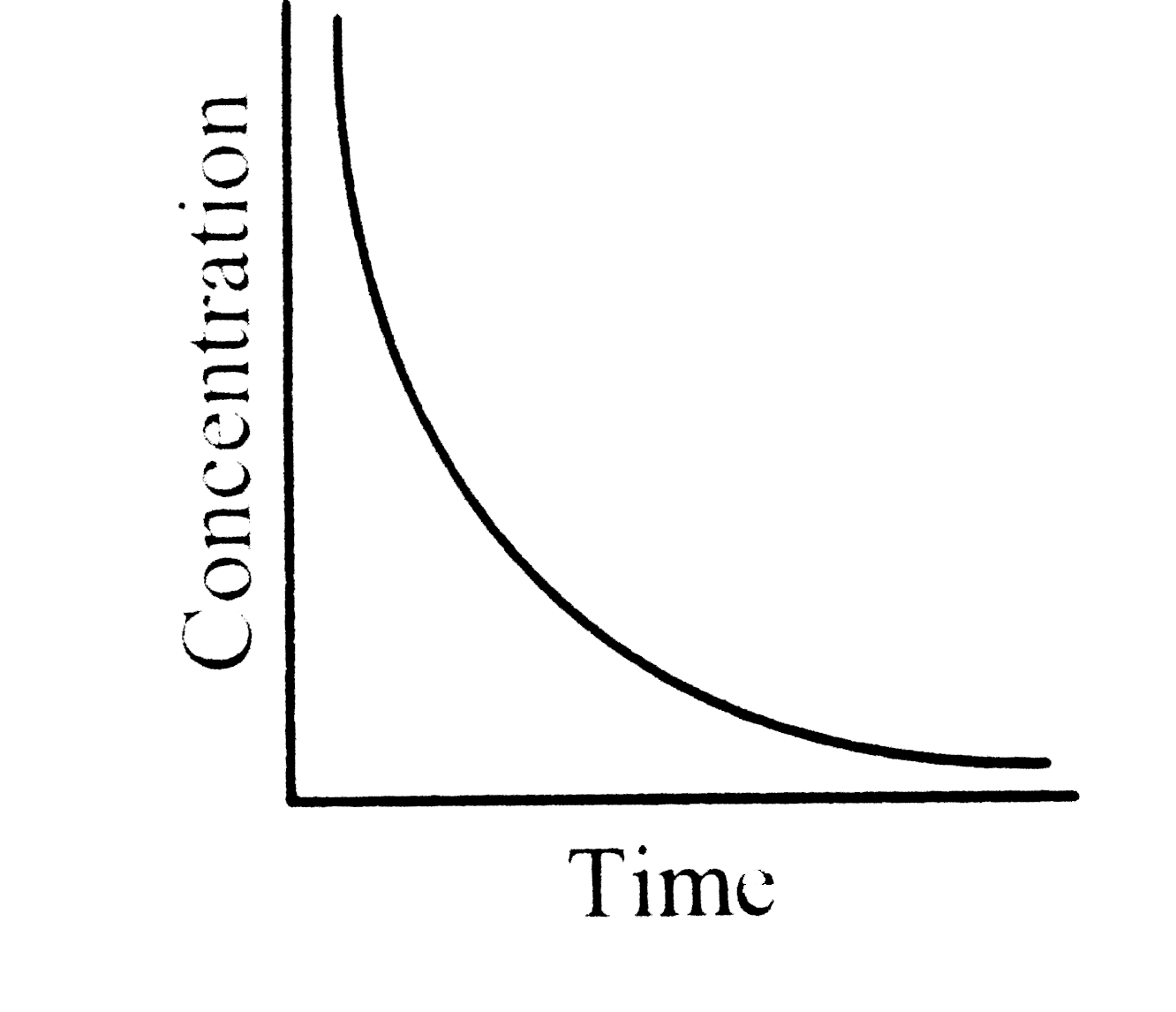
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Certain reactions follow the relation between concentrations of the re...
Text Solution
|
- The plot of concentration of the reactant vs time for a reaction is a ...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- Which among the following plots are linear (a -x) is the concentration...
Text Solution
|
- Certain reactions follow the relation between concentrations of the re...
Text Solution
|
- Analyse the given graph,drawn between concentration of reactant vs. ti...
Text Solution
|
- The plot of a concentration of the reactant vs time for a reaction is ...
Text Solution
|
- For a zero order reaction, the initial concentration of the reactant =...
Text Solution
|