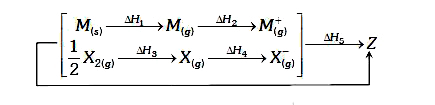A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the Born- Haber cycle for the formation of an ionic compound ...
Text Solution
|
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- How will you determine the lattice energy of an ionic compound using B...
Text Solution
|
- Write down the Born-Haber cycle for the formation of CaCl2
Text Solution
|
- Write the Born-Haber cycle for NaCl.
Text Solution
|
- Explain the steps involved in Born Haber cycle for the formation of Na...
Text Solution
|
- Write down the Born-Haber cycle for the formation of CaCl2
Text Solution
|
- Consider the Born - Haber cycle for the formation of a ionic compound ...
Text Solution
|