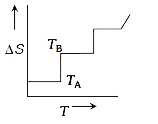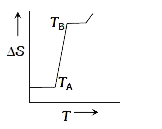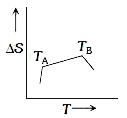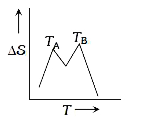A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If for a given substance, melting point is T(B) and freezing point is ...
Text Solution
|
- Which of the following graphs best illustrates the variation of entrop...
Text Solution
|
- In following isothermal graphs A, B and C at temperatures T(1), T(2) a...
Text Solution
|
- If for a given substance, melting point is T(B) and freezing point is ...
Text Solution
|
- DeltaH("fus") is the latent heat of melting and T("fus") is the meltin...
Text Solution
|
- if for a given substance , melting point is T(B) and freezing point is...
Text Solution
|
- If for a given substance, melting point is T(B) and freezing point is ...
Text Solution
|
- In the P - V diagram, the point B and C correspond to temperatures T (...
Text Solution
|
- When the same quantity of heat is absorbed by a system at teo differen...
Text Solution
|