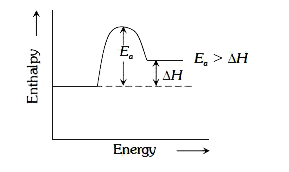A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- For an endothermic reaction energy of activation is E(a) and enthlpy o...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- For an endothermic reaction, where DeltaH represents the enthalpy of t...
Text Solution
|
- For endothermic reaction where DeltaH The enthalpy of the reaction sho...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- For an endothermic reaction where, Delta H represent the enthalpy of t...
Text Solution
|