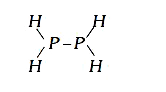A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The heat of atomisation of PH(3) is 228 K cal/mol and that os P(2)H(4)...
Text Solution
|
- The heat of atomisation of PH(3) is 228 K cal/mol and that os P(2)H(4)...
Text Solution
|
- Heat of atomisation of NH(3) and N(2)H(4) are "x kcal mol"^(-1) and "...
Text Solution
|
- The heat of atomisation of PH(3) is 228 K cal/mol and that os P(2)H(4)...
Text Solution
|
- The heat of atomisation of PH(3)(g) and P(2)H(4)(g) are 954 kJ mol^(-1...
Text Solution
|
- the heat of atomosaton pf P(4)H(4)(g)and PH(3) (g) are 355 kcal / mol ...
Text Solution
|
- The average energy required to break a P-P bond in P(4)(s) into gaseou...
Text Solution
|
- The heat of atomization of PH(3) (g) is 228 k cal mol^(-1) and that of...
Text Solution
|
- The heat of atomisation of PH(3)(g) is 228 kcal perr mol annd that of ...
Text Solution
|