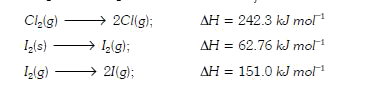A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- The enthaplpy changes state for the following processes are listed bel...
Text Solution
|
- The enthalpy change for the following process are listed below: (a) ...
Text Solution
|
- The enthaplpy changes state for the following processes are listed bel...
Text Solution
|
- The reaction obey I order with respect to H(2) and ICl both. H(2) (g) ...
Text Solution
|
- The enthalpy changes for the following process are listed below : Cl(2...
Text Solution
|
- If heat of formation of HI in the given reactions (i) H(2)(g) + I(2) t...
Text Solution
|
- The standard enthalpy of formation of H(2)(g) and Cl(2)(g) and HCl(g) ...
Text Solution
|
- The standard enthalpy of formation of H(2)(g) and Cl(2)(g) and HCl(g) ...
Text Solution
|
- The enthalpy change for the following processes are listed below {:(Cl...
Text Solution
|