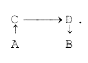A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The direct conversion of A or B is difficult, hence it is carried out ...
Text Solution
|
- The direct conversion of A to B is difficult, hence it is carried out ...
Text Solution
|
- Free energy change ( DeltaG) is related to the total entropy change (D...
Text Solution
|
- The direct conversion of A to B is difficult. Hence, it is carried out...
Text Solution
|
- Which formulate in the following are correct? a) G=H-TS b) DeltaG(...
Text Solution
|
- In the cyclic process A overset(I)toBoverset(II)Coverset(III)toA the c...
Text Solution
|
- In a reversible process, DeltaS("system") + DeltaS("surrounds") is:
Text Solution
|
- The direct conversion of A or B is difficult, hence it is carried out ...
Text Solution
|
- A का B में सीधा परिवर्तन बहुत कठिन है। अतः इस परिवर्तन को निम्न प्रकार...
Text Solution
|