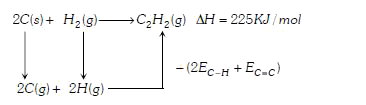A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Using the data provided, calculate the multiple bond energy (kJ "mol"...
Text Solution
|
- H(2)(g) +(1)/(2)O(2)(g) rarr H(2)O(g) DeltaH =- 242 kJ mol^(-1) Bond...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- The average O-H Bond energy in H(2)O with the help of following data :...
Text Solution
|
- Given C(2)H(2)(g)+H(2)(g)rarrC(2)H(4)(g): DeltaH^(@)=-175 " kJ mol"^(-...
Text Solution
|
- Calculate DeltaH for the reaction H(2)(g)+1//2O(2)(g)toH(2)O(g) gi...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- Using the data provided, calculate the bond energy ( kJ mol^(-1)) of a...
Text Solution
|
- Using the data provided, calculate the mutliple bond energy (kJ mol^(-...
Text Solution
|