A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
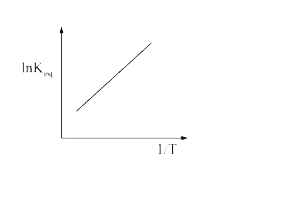
- A schematic plot of ln K(eq) versus inverse of temperature for a react...
Text Solution
|
- A schematic plot of log K(eq) vs inverse of temperature for a reaction...
Text Solution
|
- The graph relates. In K(eq.) vs.(1)/(T) for a reaction. The reaction m...
Text Solution
|
- A schematic plot of In K(eq) versus inverse o ftemperature for a react...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- Plot ln K vs. 1/T for two reactions A and B are given below. Which rea...
Text Solution
|
- A schematic plot of ln K(eq) versus inverse of temperature for a react...
Text Solution
|
- A schematic plot of ln K(eq) versus inverse of temperature for a react...
Text Solution
|
- A schematic plot of In k(eq) versus inverse of temperature for a react...
Text Solution
|