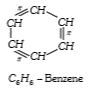A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Each carbon atom in benzene is in the state of hybridization
Text Solution
|
- एक कार्बोकैटायन में केंद्रीय कार्बन परमाणु की संकरण अवस्था बताइए ।
Text Solution
|
- बेंजीन में कार्बन परमाणुओं की संकरण अवस्था क्या है?
Text Solution
|
- In benzene, each carbon atom undergoes
Text Solution
|
- Indicate the hybrid state of each carbon atom in compounds - CH2 = C =...
Text Solution
|
- যৌগগুলিতে প্রতিটি কার্বন পরমাণুর সংকরায়িত অবস্থা নির্দেশ করো - CH3-CH...
Text Solution
|
- Indicate the hybrid state of each carbon atom in compounds - (CH3) 2CO
Text Solution
|
- যৌগগুলিতে প্রতিটি কার্বন পরমাণুর সংকরায়িত অবস্থা নির্দেশ করো - CH2=CH...
Text Solution
|
- যৌগগুলিতে প্রতিটি কার্বন পরমাণুর সংকরায়িত অবস্থা নির্দেশ করো - C6H6
Text Solution
|