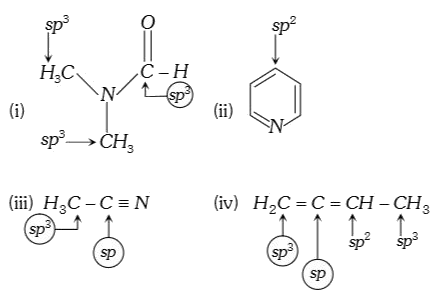
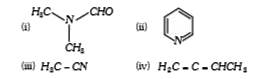A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compounds containing sp hybridized carbon atom are (iii) H(3...
Text Solution
|
- What hybrid orbitals will form the following compound H(3)C-CH=CH-CH(2...
Text Solution
|
- Which hybrid orbitals are used by the carbon atoms in the following mo...
Text Solution
|
- What are the hydridised states of carbon atoms in the following compou...
Text Solution
|
- Total number of sp-hybridised C-atoms in the folowing Hydrocarbon will...
Text Solution
|
- Discuss the concept of hybridisation. What are its different types in ...
Text Solution
|
- Indicate the type of hybridization of each carbon atom in the followin...
Text Solution
|
- In which of the compounds below is there more than one kind of hybridi...
Text Solution
|
- Hybridization of C(2) and C(3) of H(3)C-CH=C=CH-CH(3) are:
Text Solution
|