A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
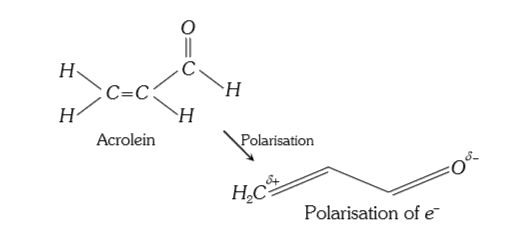
- Polarisation of electrons in acrolein may be written as :
Text Solution
|
- Polarisation of electrons in acrolein may be written as :
Text Solution
|
- Polarization of electrons in acrolein may be written as:
Text Solution
|
- Polarisation of electrons in acrolein cannot be written as :
Text Solution
|
- Polarisation of electrons in acrolein may be written as :
Text Solution
|
- Polarisation of electrons in acrolein may be written as :
Text Solution
|
- Electronic configuration calcium atom can be written as
Text Solution
|
- Polarization of electrons in acrolein may be written as:
Text Solution
|
- Polarisation in acrolein can be described as
Text Solution
|