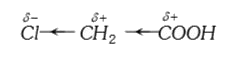A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Chloroacetic acid is a stronger acid than acetic acid this can be expl...
Text Solution
|
- Chloroacetic acid is a stronger acid than acetic acid this can be expl...
Text Solution
|
- Chloroacetic acid is a stronger acid than acetic acid. This can be exp...
Text Solution
|
- Chloroacetic acid is a stronger acid than acetic acid this can be expl...
Text Solution
|
- Chloroacetic acid is stronger than acetic acid due to
Text Solution
|
- क्लोरोऐसीटिक अम्ल, ऐसीटिक अम्ल से प्रबल होता है क्यों ?
Text Solution
|
- (b) Explain why chloroacetic acid is a stronger acid than acetic acid.
Text Solution
|
- Explain the following: (i) Benzoic acid is weeker acid than acetic aci...
Text Solution
|
- Chloroacetic acid is a stronger acid than acetic acid. This can be exp...
Text Solution
|