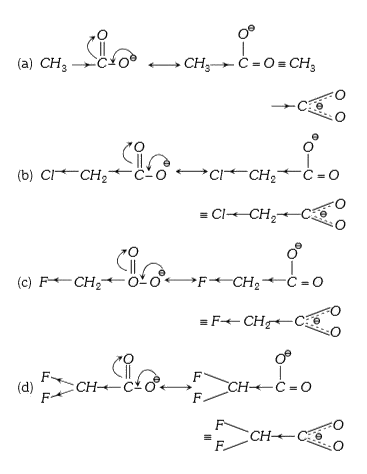
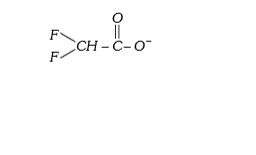A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ionic species are stabilised by the dispersal of charge. Which of the ...
Text Solution
|
- Ionic species are stabilised by the dispersal of charge which of the f...
Text Solution
|
- Ionic species are stailised by the dispersal of charge. Which of the f...
Text Solution
|
- Among the following sets, the most stable ionic species are
Text Solution
|
- Ionic species are stabilised by the dispersal of charge. Which of the ...
Text Solution
|
- Ionic species are stabilised by the dispersal of charge. Which of the ...
Text Solution
|
- The stabilization of coordination compound due to chelation is called ...
Text Solution
|
- The stabilisation of coordination compounds due to chelation is called...
Text Solution
|
- The stabilisation of coordination compounds due to chelation is called...
Text Solution
|