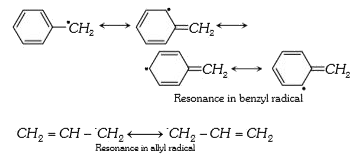
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following free radiacals in order of decreasing stability....
Text Solution
|
- Arrange the following compounds in the order of decreasing acidity. ...
Text Solution
|
- Arrange the following free radicals in the order of decreasing stabili...
Text Solution
|
- Arrange the following free radicals in order of stability underset(I)u...
Text Solution
|
- Arrange the following free radicals in decreasing order of stability:
Text Solution
|
- Arrange the following free radicals in order of decreasing stability: ...
Text Solution
|
- Arrange the following free radiacals in order of decreasing stability....
Text Solution
|
- Arrange the following free radicals in order of decreasing stability M...
Text Solution
|
- Order of stability of vinyl , allyl, teriary radicals is
Text Solution
|