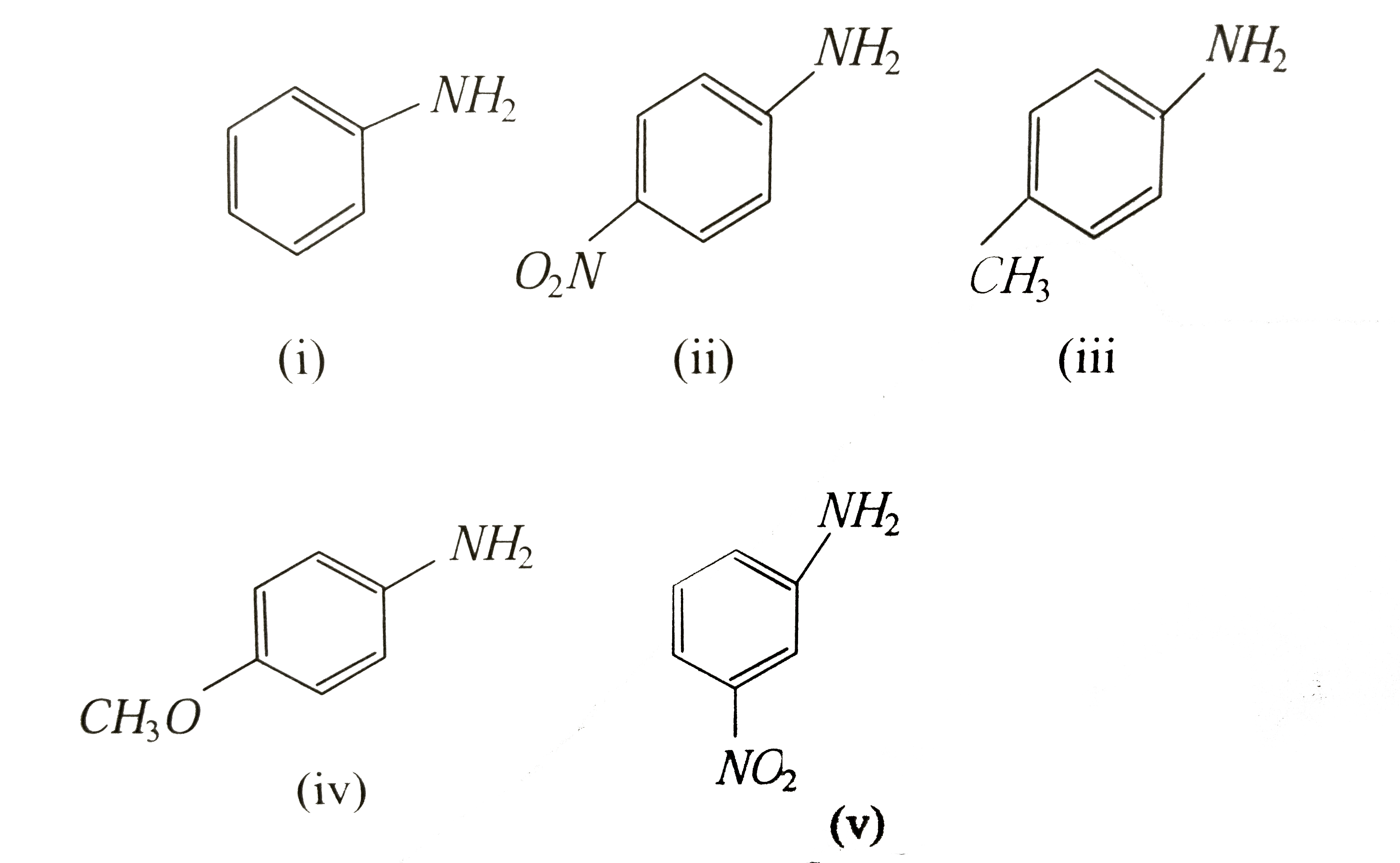
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The correct order of increasing basic nature of the following bases is
Text Solution
|
- Correct order of basic nature is
Text Solution
|
- The correct order of basic nature is
Text Solution
|
- The correct order of increasing basic nature of the bases NH(3),CH(2)N...
Text Solution
|
- The correct order of increasing basic nature of the following bases is...
Text Solution
|
- The correct order of increasing basic nature for the bases NH(3) CH(3)...
Text Solution
|
- The correct order of increasing basic nature of the following bases is
Text Solution
|
- Arrange the following bases in increasing order of their basic strengt...
Text Solution
|
- To correct the order of basic nature is
Text Solution
|