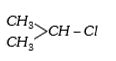A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- S(N)1 reaction is faster in
Text Solution
|
- S(N)1 reaction is faster in
Text Solution
|
- S(N^(1))and S(N^(2)) reactions are
Text Solution
|
- Which of the following compounds will react faster in S(N^(1)). Reacti...
Text Solution
|
- Which will react faster in S(N^(1)) displacement reaction? 1-Bromope...
Text Solution
|
- (a) Which has faster rate of S(N^(1))? (b) Which has faster rate ...
Text Solution
|
- Which of the following haloalkanes would undergo S(N^(2)) reaction fas...
Text Solution
|
- Which will undergo S(N^(2)) reaction faster
Text Solution
|
- Statement -1 : Rate of S(N)1 reaction is faster than that of S(N)2 rea...
Text Solution
|