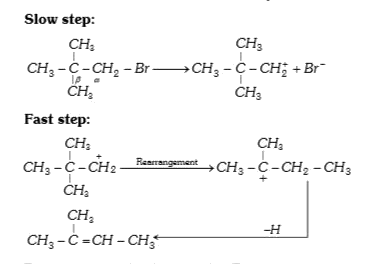
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Neopentyl bromide undergoes dehydro halogenation to give alkene even t...
Text Solution
|
- Neopentyl bromide is heated with an alcoholic KOH solution. The major ...
Text Solution
|
- Neopentyl bromide undergoes dehydro halogenation to give alkene even t...
Text Solution
|
- Which of the following compound does not undergo base-ctalyzed exchang...
Text Solution
|
- An alkyl bromide produces a single alkene when it reacts with sodium e...
Text Solution
|
- Neopentyl bromide undergoes dehydrohalogenation to give alkene even th...
Text Solution
|
- Neopentyl bromide undergoes dehydrohalogenation to give alkene even th...
Text Solution
|
- The dehydrohalogenation of neopentyl bromide with alcoholic KOH mainly...
Text Solution
|
- किसी 1,2- डाइहैलाइड का डीहाइड्रो हैलोजनीकरण करने पर एक प्राप्त होता ह...
Text Solution
|