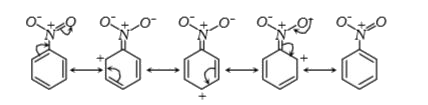
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In electrophilic aromatic substitution reaction, the nitro group is me...
Text Solution
|
- Which of the following is not a meta-directing group in electrophilic ...
Text Solution
|
- Meta-directing and deactivating group in the aromatic electrophilic su...
Text Solution
|
- In electrophilic aromatic substitution reaction, the nitro group is me...
Text Solution
|
- Meta-directing and deactivating group in aromatic electrophilic substi...
Text Solution
|
- In an electrophilic substitution reaction of nitrobenzene, the presenc...
Text Solution
|
- In electrophilic aromatic substitution reaction, the nitro group is me...
Text Solution
|
- Which of the following is not meta-directing group in electrophilic ar...
Text Solution
|
- In electrophilic aromatic substitution reaction, the nitro group is me...
Text Solution
|